David Longmuir of Phillips Ormonde Fitzpatrick Lawyers and Mark Wickham of Phillips Ormonde Fitzpatrick recently acted for Rozenberg & Co in successfully opposing the grant of Australian Patent Application 2010294197 for a “method for the preparation of micro-RNA and its therapeutic application” in the name of Velin-Pharma A/S – see Rozenberg & Co Pty Ltd v Velin-Pharma A/S [2017] (‘Rozenberg’).
The decision is notable for its application of the grace period provided in paragraph 24(1)(a) of the Act. Subsection 24(1) states:
“24 Validity not affected by certain publication or use
(1) For the purpose of deciding whether an invention is novel or involves an inventive step or an innovative step, the person making the decision must disregard:
(a) any information made publicly available, through any publication or use of the invention in the prescribed circumstances, by or with the consent of the nominated person or patentee, or the predecessor in title of the nominated person or patentee; and
(b) any information made publicly available without the consent of the nominated person or patentee, through any publication or use of the invention by another person who derived the information from the nominated person or patentee or from the predecessor in title of the nominated person or patentee;
but only if a patent application for the invention is made within the prescribed period.”
The Opponent argued that the grace period provisions did not apply to a relevant whole of contents prior art document (D13) in circumstances where it was published after the filing date of the opposed application (that is, after the grace period had ended).
In Rozenberg, the date of publication of D13 (21 October 2010), which has an earlier priority date than the Opposed Application, was after the filing date of the opposed application (10 September 2010).
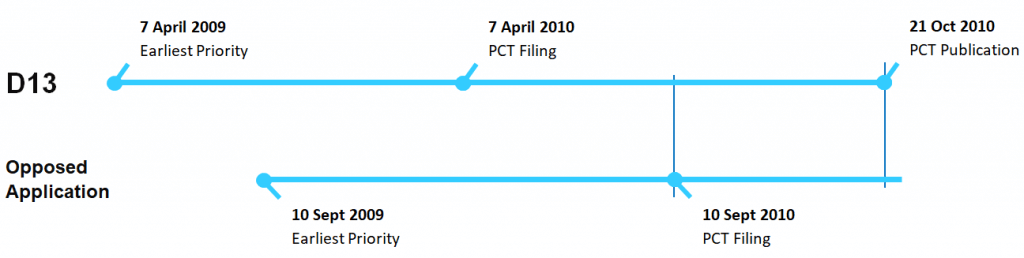
The regulations (2.2(1A)) provided for an application of the grace period where “there was a publication or use of the invention within 12 months before the filing date of the complete application”. The Opponent argued that because the date of publication of D13 was after (not before) the filing date of the opposed application, the grace period provisions are not available to disregard D13.
However, the Delegate relevantly found that subregulation 2.2(1A) sets a starting but not final date for the relevant period for publication, despite the regulation explicitly requiring that the publication be within 12 months before the filing date of the complete application.
Having considered that subregulation 2.2(1A) also applies to publications made after the filing date of the complete application, the Delegate then considered the application of subregulation 2.3(1A) which provides that where the applicant relies on 2.2 (1A), “the prescribed period is… 12 months after the information was first made publicly available.”
Applying the same logic to the interpretation of subregulation 2.3(1A), the Delegate found that the regulation does not specify a start date, and therefore the filing of the opposed application took place before the relevant period was completed. On this basis, the filing of the opposed application was considered to meet the requirements of 2.3(1A) and therefore the grace period provisions apply. D13 was therefore set aside from any consideration of novelty.
Despite the Delegate indicating that the approach was consistent with that taken in Biogen Idec MA Inc, the decision is likely to benefit patent applicants as it broadens the previous understanding of the applicability of the grace period provisions.
